Part A: After the reaction, approximately 7.08g of acetic acid is present.
Part B: After the reaction, approximately 5.46g of lead(II) sulfate is present.
Part C: After the reaction, the same amount of lead(II) sulfate (5.46g) is present.
Part D: After the reaction, approximately 7.08g of acetic acid is present.
Part A: Calculating the grams of sulfuric acid present after the reaction.
The balanced chemical equation for the reaction is:
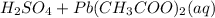 →
→
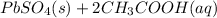
The molar mass of sulfuric acid (
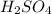 )98.08g/mol. Using the given mass of sulfuric acid (5.80g), we can calculate the moles of sulfuric acid:
)98.08g/mol. Using the given mass of sulfuric acid (5.80g), we can calculate the moles of sulfuric acid:
Moles of
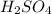 =
=
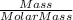 =
=
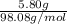
Moles of
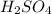 ≈0.059mol
≈0.059mol
After the reaction, every mole of sulfuric acid produces one mole of acetic acid. So, the moles of acetic acid formed (2 × Moles of 2×Moles of
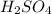 ):
):
Moles of acetic acid =2×0.059 mol
Now, we can find the mass of acetic acid
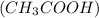 using its molar mass (60.05g/mol):
using its molar mass (60.05g/mol):
Mass of acetic acid = Moles of acetic acid × Molar Mass of acetic acid
Mass of acetic acid=2×0.059mol×60.05g/mol
Mass of acetic acid ≈ 7.08g
Part B: Calculating the grams of lead(II) acetate present after the reaction.
The molar mass of lead(II) acetate (
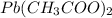 ) is 325.29g/mol. Using the given mass of lead(II) acetate (5.80g), we can calculate the moles of lead(II) acetate:
) is 325.29g/mol. Using the given mass of lead(II) acetate (5.80g), we can calculate the moles of lead(II) acetate:
Mass Molar Mass = 5.80 g 325.29 g/mol
Moles of
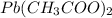 =
=
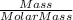 =
=
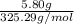
Moles of
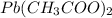 ≈0.018mol
≈0.018mol
After the reaction, every mole of lead(II) acetate produces one mole of lead(II) sulfate(
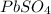 ). So, the moles of lead(II) sulfate formed:
). So, the moles of lead(II) sulfate formed:
Moles of
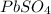 =0.018mol
=0.018mol
Now, we can find the mass of lead(II) sulfate using its molar mass
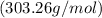 :
:
Mass of
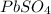 =Moles of
=Moles of
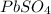 ×Molar Mass of
×Molar Mass of
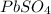
Mass of
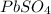 =0.018mol×303.26g/mol
=0.018mol×303.26g/mol
Mass of
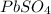 ≈5.46g
≈5.46g
Part C: Calculating the grams of lead(II) sulfate present after the reaction.
The moles of lead(II) sulfate formed (0.018mol) are the same as calculated in Part B.
Part D: Calculating the grams of acetic acid present after the reaction.
The mass of acetic acid (7.08g) is the same as calculated in Part A.