Answer:
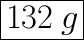
Step-by-step explanation:
To find the mass of the molecule given it's number of moles, we first find the molar mass of
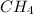 after that we use the formula;
after that we use the formula;
mass(g) = Molar mass (g/mol) × moles
Molar mass of
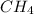 = 12 +4(1) = 12+4 = 16 g/mol
= 12 +4(1) = 12+4 = 16 g/mol
number of moles = 8.25 mol
mass = 16 × 8.25 = 132
We have the final answer as
132 g