1. 4.00 moles of
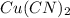 corresponds to
corresponds to
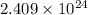 atoms or molecules.
atoms or molecules.
2. 70.9 moles of Au corresponds to
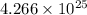 atoms or molecules.
atoms or molecules.
3.
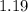 moles of
moles of
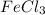 corresponds to
corresponds to
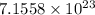 atoms or molecules.
atoms or molecules.
To find the number of atoms or molecules from the number of moles, you can use Avogadro's number, which is approximately
 particles per mole. The formula to convert moles to atoms or molecules is:
particles per mole. The formula to convert moles to atoms or molecules is:
![\[ \text{Number of particles} = \text{Number of moles} * \text{Avogadro's number} \]](https://img.qammunity.org/2024/formulas/chemistry/college/g8yo0qgzlg7ncz6wybwoh2ywfyjuikx7m9.png)
1. For
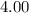 moles of
moles of
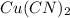 :
:
![\[ \text{Number of atoms or molecules} = 4.00 \, \text{moles} * 6.022 * 10^(23) \, \text{particles/mole} \]](https://img.qammunity.org/2024/formulas/chemistry/college/xn02x7fvzfp2jw75boogjpg5eu8798qxjf.png)
![\[ \text{Number of atoms or molecules} = 2.409 * 10^(24) \, \text{atoms or molecules} \]](https://img.qammunity.org/2024/formulas/chemistry/college/bp2de1ze4gk2wlrhcpq1fxk8mj57z9xu5g.png)
2. For
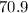 moles of
moles of
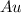 :
:
![\[ \text{Number of atoms or molecules} = 70.9 \, \text{moles} * 6.022 * 10^(23) \, \text{particles/mole} \]](https://img.qammunity.org/2024/formulas/chemistry/college/h4m2r6eim5n0aaxplm5ft6wv1dygv660aa.png)
![\[ \text{Number of atoms or molecules} = 4.266 * 10^(25) \, \text{atoms or molecules} \]](https://img.qammunity.org/2024/formulas/chemistry/college/b0m18mn1bsznz8xp5r3g3sp0miaudpgqu0.png)
3. For
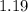 moles of
moles of
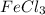 :
:
![\[ \text{Number of atoms or molecules} = 1.19 \, \text{moles} * 6.022 * 10^(23) \, \text{particles/mole} \]](https://img.qammunity.org/2024/formulas/chemistry/college/flicu8e5412yu2q74zky2utpk2wamosac5.png)
![\[ \text{Number of atoms or molecules} = 7.1558 * 10^(23) \, \text{atoms or molecules} \]](https://img.qammunity.org/2024/formulas/chemistry/college/xy63vrynxxmgo2kkzrzlv59rm5deiku1j3.png) .
.