Answer:

Step-by-step explanation:
Percent yield is the ratio of the amount actually produced to how much could theoretically be produced. It is found using this formula:
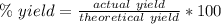
For this reaction, the theoretical or expected yield is 325.0 grams. The actual yield is 123.8 grams.
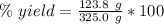
Divide.
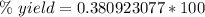
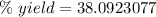
Round to the nearest hundredth. The 9 in the hundredth place tells us to round the 0 to a 1 .
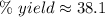
The percent yield is about 38.1%