Final Answer:
The balanced chemical reaction for the combustion of octane (C8H18) is:
![\[2 \text{C}_8\text{H}_(18(l)) + 25 \text{O}_2(g) \rightarrow 16 \text{CO}_2(g) + 18 \text{H}_2\text{O}(l)\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/5e6762b6lnd2qbuh6xpr61j8u2h9yb7x9x.png)
Step-by-step explanation:
Octane
 combustion is a chemical reaction with oxygen
combustion is a chemical reaction with oxygen
 producing carbon dioxide
producing carbon dioxide
 and water
and water
 as products. The balanced equation is
as products. The balanced equation is
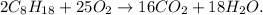
In this equation, the coefficients ensure that the number of each type of atom is the same on both sides of the reaction, maintaining the law of conservation of mass. The balanced reaction signifies that two moles of octane react with 25 moles of oxygen to yield 16 moles of carbon dioxide and 18 moles of water.
This reaction is essential in understanding the combustion process of hydrocarbons, crucial for applications like internal combustion engines in vehicles.
The combustion of hydrocarbons is a fundamental concept in chemistry, and understanding these reactions is crucial for various industrial and environmental applications. It provides insights into energy release, emission control, and the efficient use of fuel sources.