The boiling points of compounds depend on the strength of the intermolecular forces (IMFs). Iodine has stronger dispersion forces than bromine, resulting in a higher boiling point for I₂. HF forms hydrogen bonding, while HCl has weaker dipole-dipole forces, resulting in a higher boiling point for HF. Similarly, bromine has stronger dispersion forces than chlorine, giving
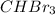 a higher boiling point than
a higher boiling point than
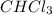 .
.
The boiling points of compounds are determined by the strength of the intermolecular forces (IMFs) between their molecules. In general, the stronger the IMFs, the higher the boiling point.
For example, in comparing the boiling points of molecular pairs:
- a.
 (59°C) and I2 (184°C): The IMFs in I2 are stronger than those in
(59°C) and I2 (184°C): The IMFs in I2 are stronger than those in
 because iodine atoms are larger and have more electrons, resulting in stronger dispersion forces. Therefore, I2 has a higher boiling point than
because iodine atoms are larger and have more electrons, resulting in stronger dispersion forces. Therefore, I2 has a higher boiling point than
 .
. - b. HF (20°C) and HCl (-85°C): The boiling point of HF is higher than that of HCl because HF can form hydrogen bonding, which is a very strong dipole-dipole interaction. HCl only has weaker dipole-dipole forces. Therefore, HF has a higher boiling point than HCl.
- c.
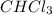 (61°C) and
(61°C) and
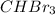 (150°C): The boiling point of
(150°C): The boiling point of
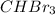 is higher than that of
is higher than that of
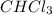 because bromine atoms are larger and have more electrons, resulting in stronger dispersion forces. Therefore,
because bromine atoms are larger and have more electrons, resulting in stronger dispersion forces. Therefore,
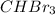 has a higher boiling point than
has a higher boiling point than
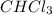 .
.