The sum of the oxidation states of Mn and N is smallest in Row A and Row D.
Let's do the calculation for each row.
Row A:
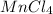 and
and

Oxidation state of Mn in
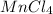 is +2.
is +2.
Oxidation state of N in
 is 0.
is 0.
Sum = +2 + 0 = +2
Row B:
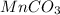 and
and
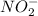
Oxidation state of Mn in
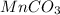 is +2.
is +2.
Oxidation state of N in
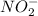 is +4.
is +4.
Sum = +2 + +4 = +6
Row C:
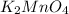 and NH4+
and NH4+
Oxidation state of Mn in
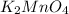 is +6.
is +6.
Oxidation state of N in NH4+ is -3.
Sum = +6 + -3 = +3
Row D:
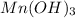 and
and
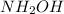
Oxidation state of Mn in
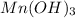 is +3.
is +3.
Oxidation state of N in
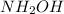 is -1.
is -1.
Sum = +3 + -1 = +2
Therefore, the sum of the oxidation states of Mn and N is smallest in Row A and Row D.