The reaction is exothermic because the energy of
 and
and
 is less than the energy of
is less than the energy of
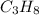 and
and
 . Option C.
. Option C.
In an exothermic reaction, the overall reaction releases energy, usually in the form of heat. The reactants have a higher potential energy compared to the products, and the reaction proceeds by overcoming the activation energy barrier, leading to the formation of lower-energy products.
The products have lower potential energy and are more stable than the reactants, and the overall reaction releases energy to the surroundings.
In the diagram, the energy of the products,
 and
and
 , is less than the energy of the reactants,
, is less than the energy of the reactants,
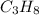 and
and
 . Thus, the reaction is exothermic.
. Thus, the reaction is exothermic.