The rate of the ammonia synthesis reaction when [N₂] = 0.0154 M is approximately 0.0025 M/s.
We notice that doubling the [H₂] concentration (from experiment 2 to 3) while keeping [N₂] constant does not affect the reaction rate and a suggestion that the reaction is first-order in N₂ and zero-order in H₂.
The reaction order and rate constant remain constant at different concentrations.
Rate = k * [N₂]

0.03759 M/s = k * (0.231 M)

k = 0.1628
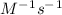
We find the Rate at [N₂] = 0.0154 M:
Rate = k * [N₂]

Rate = 0.1628
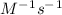 * (0.0154 M)
* (0.0154 M)

Rate = 0.0025 M/s