The electrode on the left will be positive. Using the Nernst equation with standard cell potential, concentrations, and temperature, the voltmeter will show approximately 0.02 V, assuming
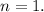
The electrode on the left will be positive in this galvanic cell configuration. The movement of electrons from the left half-cell to the right half-cell generates a potential difference, making the left electrode positive and the right electrode negative.
To determine the voltage, we can use the Nernst equation for calculating the cell potential (
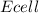 ) under non-standard conditions:
) under non-standard conditions:
![\[Ecell = E^o - (0.0592)/(n) \cdot \log\left(([M^+]_2^n)/([M^+]_1^n)\right)\]](https://img.qammunity.org/2024/formulas/chemistry/college/nexy21tgb3gxjjxmnc1nnsrrebzi3fjzdc.png)
Here,
 is the standard cell potential,
is the standard cell potential,
![\([M^+]_2\) and \([M^+]_1\)](https://img.qammunity.org/2024/formulas/chemistry/college/mcyc0mzps7dpiqqvuyojqlda1o1uq1ignp.png) are the molar concentrations of
are the molar concentrations of
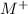 in the right and left half-cells, and \(n\) is the number of moles of electrons transferred (which is equal to the number of ions of
in the right and left half-cells, and \(n\) is the number of moles of electrons transferred (which is equal to the number of ions of
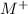 involved).
involved).
Since the right and left half-cells contain the same substance, \(n\) is likely equal to 1. The standard cell potential
 for a galvanic cell with
for a galvanic cell with
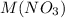 as the electrolyte can be looked up.
as the electrolyte can be looked up.
Given that the temperature is constant at 35.0 °C, the Nernst equation allows us to find the non-standard cell potential. Solving this equation will provide the voltage, and rounding the result to two significant digits will give the final answer.