The equilibrium concentration of Ni2+ in a 1.0 M solution of [Ni(NH3)6] is
 , determined by the formation constant (Kf) expression and initial conditions.
, determined by the formation constant (Kf) expression and initial conditions.
To calculate the equilibrium concentration of Ni2+ in a 1.0 M solution of [Ni(NH3)6], we can use the formation constant (Kf) expression and the stoichiometry of the reaction:
![\[ \text{Ni}^(2+) + 6\text{NH}_3 \rightleftharpoons [\text{Ni(NH}_3)_6]^(2+) \]](https://img.qammunity.org/2024/formulas/chemistry/college/wds6waf9mql22mkfer7zjhjhkxrpucczkq.png)
The formation constant expression is given as:
![\[ K_f = \frac{[\text{Ni(NH}_3)_6]^(2+)}{\text{[Ni}^(2+)][\text{NH}_3]^6} \]](https://img.qammunity.org/2024/formulas/chemistry/college/oanv7lfdfwcrp7195rtsepc2pf3dtx04jd.png)
Given that
 , and initially, there is no
, and initially, there is no
![\([\text{Ni(NH}_3)_6]^(2+)\)](https://img.qammunity.org/2024/formulas/chemistry/college/d6bnmadz8zy06djzlg3pj6hdw1gvbm804n.png) , and the concentration of
, and the concentration of
 is 1.0 M, we can set up the expression:
is 1.0 M, we can set up the expression:
![\[ 2.0 * 10^8 = (x)/((1.0)^6) \]](https://img.qammunity.org/2024/formulas/chemistry/college/mxsr0nzkl3bvoocv4gpnvg6urx2zeebe7g.png)
Solving for
 , the equilibrium concentration of
, the equilibrium concentration of
![\([\text{Ni(NH}_3)_6]^(2+)\)](https://img.qammunity.org/2024/formulas/chemistry/college/d6bnmadz8zy06djzlg3pj6hdw1gvbm804n.png) :
:
![\[ x = 2.0 * 10^8 \, \text{M} \]](https://img.qammunity.org/2024/formulas/chemistry/college/jmnxmnzkysx1tdbsi136nhztoqbdqla9ph.png)
Therefore, the equilibrium concentration of
 in the solution is
in the solution is
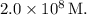
The probable question may be:
Calculate the equilibrium concentration of
 in a 1.0 M solution
in a 1.0 M solution
![[Ni(NH_3)_6]. Ni2+ (aq) + 6NH_3(aq) = [Ni(NH_3)_6]2^+(aq) Kf = 2.0 x 10^8](https://img.qammunity.org/2024/formulas/chemistry/college/ob0gaqjd7o5kirg7eblhk5zumgwmrune65.png)