The activity of the
 in 2.4 g of
in 2.4 g of
 found in living tissue is approximately 6 Bq.
found in living tissue is approximately 6 Bq.
The activity (A) of a radioactive substance can be calculated using the equation:
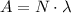
where:
- (N) is the number of radioactive atoms
 is the decay constant.
is the decay constant.
The decay constant is related to the half-life (T) of the substance by the equation:
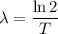
Given that the half-life of
 is 5730 years as shown in the question, the decay constant can be calculated by:
is 5730 years as shown in the question, the decay constant can be calculated by:
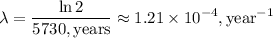
Next, we need to calculate the number of
 atoms in 2.4 g of
atoms in 2.4 g of
 . This is given by:
. This is given by:
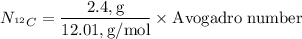
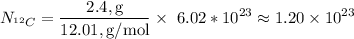
Since the fraction of
 that is
that is
 is given as
is given as
 , the number of
, the number of
 atoms is:
atoms is:
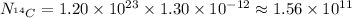
Finally, we can calculate the activity:
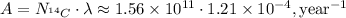
To convert this to Bq (decays per second), we need to multiply by the number of seconds in a year:
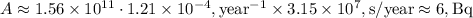
So, the activity of the
 in 2.4 g of
in 2.4 g of
 found in living tissue is approximately 6 Bq.
found in living tissue is approximately 6 Bq.