Answer:
Number of moles = 0.36 moles.
Step-by-step explanation:
Given the following data;
Mass of KNO3 = 36.4g
To find the number of moles;
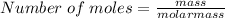 .....equation 1.
.....equation 1.
Molar mass of KNO3;
Molecular weight of potassium, K = 39g/mol.
Molecular weight of nitrogen, N = 14g/mol.
Molecular weight of oxygen, O3 = 16*3 = 48g/mol.
Therefore, molar mass of KNO3 = 39 + 14 + 48 = 101g/mol
Substituting the values into eqn 1, we have;
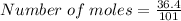
Number of moles = 0.36 moles.