Answer:
2,47 *
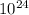 (molecules)
(molecules)
Step-by-step explanation:
Avogadro's number is a very important relationship: 1 mole = 6,02 *
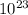 atoms, molecules, protons, etc.
atoms, molecules, protons, etc.
To convert from moles to molecules, multiply the molar amount by Avogadro's number.
4,1 mol * 6,02 *
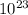 ≈ 24,7 *
≈ 24,7 *
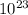 = 2,47 *
= 2,47 *
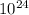 (molecules)
(molecules)
Therefore the third answer is correct