Answer:
The new volume of the gas is 9.086 liters.
Step-by-step explanation:
Let suppose that nitrogen has a behavior of ideal gas, the equation of state for ideal gases is:
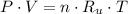 (1)
(1)
Where:
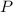 - Pressure, measured in atmospheres.
- Pressure, measured in atmospheres.
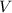 - Volumen, measured in liters.
- Volumen, measured in liters.
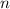 - Molar amount, measured in moles.
- Molar amount, measured in moles.
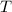 - Temperature, measured in Kelvin.
- Temperature, measured in Kelvin.
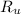 - Ideal gas constant, measured in atmosphere-liters per mole-Kelvin.
- Ideal gas constant, measured in atmosphere-liters per mole-Kelvin.
If pressure and molar amount of the gas remain constant, then we construct the following relationship:
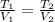 (2)
(2)
If we know that
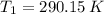 ,
,
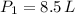 and
and
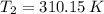 , then the new volume of the gas is:
, then the new volume of the gas is:
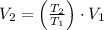
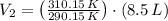
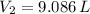
The new volume of the gas is 9.086 liters.