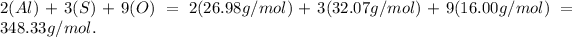Final answer:
The formula for aluminum sulfite is
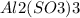 . It contains
. It contains
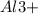 and
and
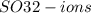 combined in a
combined in a
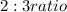 . The formula mass of this compound is
. The formula mass of this compound is
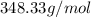 .
.
Step-by-step explanation:
The formula for aluminum sulfite is
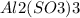 (option B).
(option B).
The formula indicates that it contains
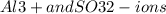 combined in .
combined in .
For purposes of computing a formula mass it is helpful to rewrite the formula in the simpler format
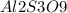 .
.
The formula mass for this compound is calculated as follows: