The mass of the sodium ethoxide is 7.4 g.
What is stoichiometry?
In stoichiometry, the coefficients in a balanced chemical equation represent the mole ratios between different substances. By understanding these ratios, one can determine the amount of reactants needed or products formed in a chemical reaction.
Number of moles of
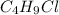 = 6.83 g /93 g/mol
= 6.83 g /93 g/mol
= 0.073 moles
Since the reaction is 1: 1, to have a 50% excess we should have half the concentration of
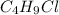 which is 0.073 moles + 0.0365 = 0.1095 moles
which is 0.073 moles + 0.0365 = 0.1095 moles
Mass of the
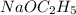 = 0.1095 moles * 68 g/mol
= 0.1095 moles * 68 g/mol
= 7.4 g
Missing parts;
The following reaction is stoichiometric as written
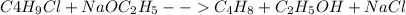 but it is often carried out with an excess of
but it is often carried out with an excess of
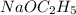 to react with any water present in the reaction mixture that might reduce the yield. If the reaction shown was carried out with 6.83 g of
to react with any water present in the reaction mixture that might reduce the yield. If the reaction shown was carried out with 6.83 g of
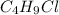 , how many grams of
, how many grams of
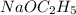 would be needed to have a 50 percent molar excess of the reactant?
would be needed to have a 50 percent molar excess of the reactant?