The temperature change is obtained as 2.5. Option A
What is the heat of solution?
The heat of solution, also known as enthalpy of solution, refers to the heat energy absorbed or released when a substance dissolves in a solvent to form a solution. This process can be exothermic or endothermic, depending on whether heat is released or absorbed during dissolution.
The Heat given off by the solution = 210 kJ/mol * 0.0025 moles of Cu II
= 0.525 kJ or 525 J
But 50
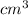 = 50 g
= 50 g
Now;
H = mcdT
dT = H/mc
dT = 525 J/50 * 4.2
dT = 2.5