The molecular formula is
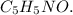
Carbon (C):
Moles of Carbon =
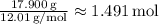
Hydrogen (H):
Moles of Hydrogen =
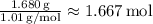
Oxygen (O):
Moles of Oxygen =
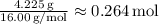
Nitrogen (N):
Moles of Nitrogen =
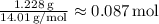
Next, find the ratio of moles:
The smallest mole value is approximately 0.087 mol (Nitrogen).
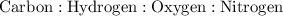
\
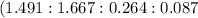
This equates to: 4 : 4 : 1 : 1
Therefore, the empirical formula is
 .
.
Now, calculate the empirical formula mass:
![\[ \text{Empirical Formula Mass} = (4 * \text{Atomic Mass of C}) + (4 * \text{Atomic Mass of H}) + \text{Atomic Mass of N} + \text{Atomic Mass of O} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/2zdilcvimcw8pucajmpt4v1rycmgrilfnh.png)
![\[ \text{Empirical Formula Mass} = (4 * 12.01) + (4 * 1.01) + 14.01 + 16.00 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/yfewgz3qomymvirk37er5t3up1wfew6txj.png)
![\[ \text{Empirical Formula Mass} = 48.04 + 4.04 + 14.01 + 16.00 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/5zccdv4g5nrxupvhavt4h54bq16r8ini1u.png)
![\[ \text{Empirical Formula Mass} = 82.09 \, \text{g/mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/xvlk2g3hidpysikv1uc8l3hvpjjhkr2xbb.png)
To find the molecular formula, we need to compare the empirical formula mass (82.09 g/mol) with the given chemical mass (110.0 g/mol).
![\[ \text{Ratio} = \frac{\text{Given Chemical Mass}}{\text{Empirical Formula Mass}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/ejupx9tncpftxiesbzk8q05twz2i2u9w3c.png)
![\[ \text{Ratio} = (110.0)/(82.09) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/bj7rjmspgrwne0q9qwc2m96rdoob8hcgoj.png) = 1.340
= 1.340
![\[ \text{Molecular Formula} = 1.340 * C_4H_4NO \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/ngt603cese22flcnmqnc0vu1h7ebo0mihg.png)
To obtain a whole number ratio, we can round the subscripts to the nearest whole number:
![\[ \text{Molecular Formula} \approx C_5H_5N_1O_1 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/w3xamlqw7ftn382hvu9ro1rfq02zbn6fdb.png)