Answer: Molar mass of
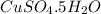 is 249.68 g/mol.
is 249.68 g/mol.
Step-by-step explanation:
Molar mass is the mass of 1 mole of the molecule. It is calculated by adding masses of all the atoms in the given molecule.
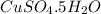 contains one atom of copper , 1 atom of sulphur, 9 atoms of oxygen and 10 atoms of hydrogen
contains one atom of copper , 1 atom of sulphur, 9 atoms of oxygen and 10 atoms of hydrogen
Mass of copper = 63.5 g/mol
Mass of sulphur = 32.06 g/mol
mass of oxygen = 16.00 g/mol
Mass of hydrogen = 1.008 g/mol
Thus mass of
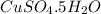 = 63.5(1)+32.06(1)+16.00(9)+1.008(10)=249.68 g
= 63.5(1)+32.06(1)+16.00(9)+1.008(10)=249.68 g
Molar mass of
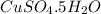 is 249.68 g/mol.
is 249.68 g/mol.