Answer:
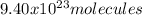
Step-by-step explanation:
Hello!
In this case, since we are given the volume of N2O3 and pressure and temperature for the STP (1.00 atm and 273.15 K), we can compute the moles, considering the ideal gas equation as shown below:
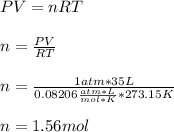
Now, by using the Avogadro's number it is possible to compute the molecules of this case in 1.56 moles:
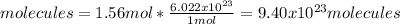
Best regards!