The hydroxide ion concentrations for pH values
a. 7.00 - 1×
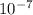 M
M
b. 11.00 - 1×
 M
M
c. 4.00 - 1×
 M
M
d. 6.00 - 1×
 M
M
To determine the hydroxide ion concentration ([
 ]) for each given pH value, we can use the formula:
]) for each given pH value, we can use the formula:
![[OH^(-)] =10^(-pH)](https://img.qammunity.org/2024/formulas/chemistry/college/v2a9jm0kl4f6wnb2kjum9yuqov8gmoas0p.png)
a. pH 7.00:
For a neutral solution with pH 7.00, the [OH^-] is calculated as
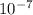 , resulting in a hydroxide ion concentration of 1×
, resulting in a hydroxide ion concentration of 1×
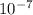 M. In neutral water, the concentration of
M. In neutral water, the concentration of
 is also 1×
is also 1×
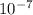 M.
M.
b. pH 11.00:
For a basic solution with pH 11.00, the
![[OH^-]](https://img.qammunity.org/2024/formulas/chemistry/college/ba3cc1je1y3fa6nvky66q3zxf35tm1ovpk.png) is calculated as
is calculated as
 . The resulting hydroxide ion concentration is 1×
. The resulting hydroxide ion concentration is 1×
 M. Basic solutions have lower
M. Basic solutions have lower
![[H^(+) ]](https://img.qammunity.org/2024/formulas/chemistry/college/5xph9l9v5c2bbql4tei0jj7qpqpa31q0ra.png) concentrations and higher
concentrations and higher
![[OH^(-)]](https://img.qammunity.org/2024/formulas/chemistry/college/gwnubwf4jngqj7hjtpw6k5f4ikzrryas3q.png) concentrations.
concentrations.
c. pH 4.00:
For an acidic solution with pH 4.00, the
![[OH^-]](https://img.qammunity.org/2024/formulas/chemistry/college/ba3cc1je1y3fa6nvky66q3zxf35tm1ovpk.png) is calculated as
is calculated as
 , resulting in a hydroxide ion concentration of 1×
, resulting in a hydroxide ion concentration of 1×
 M. Acidic solutions have higher
M. Acidic solutions have higher
![[H^(+)]](https://img.qammunity.org/2024/formulas/chemistry/college/hzjpcmspnqs9lnz8x0jv1mtouupjq6asni.png) concentrations and lower
concentrations and lower
![[OH^(-) ]](https://img.qammunity.org/2024/formulas/chemistry/college/6ubplynyy9vmjbpalk0fmjh87asw6ubuc9.png) concentrations.
concentrations.
d. pH 6.00:
For a slightly acidic solution with pH 6.00, the
![[OH^-]](https://img.qammunity.org/2024/formulas/chemistry/college/ba3cc1je1y3fa6nvky66q3zxf35tm1ovpk.png) is calculated as
is calculated as
 , resulting in a hydroxide ion concentration of 1×
, resulting in a hydroxide ion concentration of 1×
 M. The solution is more acidic than neutral but less acidic than a solution with pH 4.00.
M. The solution is more acidic than neutral but less acidic than a solution with pH 4.00.
The hydroxide ion concentrations for pH values 7.00, 11.00, 4.00, and 6.00 are 1×
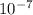 M, 1×
M, 1×
 M, 1×
M, 1×
 M and 1×
M and 1×
 M respectively.
M respectively.