For question (a) the order of increasing ionic radius is:
![\[ Ra^(2+) < Sr^(2+) < Ba^(2+) < Mg^(2+) < Be^(2+) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/mga0jtmrpsb3dyqs0xp3eo41zv7djvzemw.png) . For question (b) the complete order for increasing ionic radius is:
. For question (b) the complete order for increasing ionic radius is:
![\[ Al^(3+) < Mg^(2+) < Na^(+) < Cl^(-) < S^(2-) < P^(3-) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jt866ox8dx5rr8horjiqsf7qknfwl6ma16.png)
a) Ba²⁺, Mg²⁺, Sr²⁺, Ra²⁺, Be²⁺:
Ionic radius generally decreases as you move across a period from left to right and increases as you move down a group. This is because as you move across a period, the effective nuclear charge increases, pulling the electrons closer to the nucleus.
In contrast, moving down a group adds energy levels, resulting in larger ionic radii.
In this case, we're comparing ions with the same charge
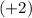 , so the size of the ions will mainly depend on the number of energy levels.
, so the size of the ions will mainly depend on the number of energy levels.
So, the order of increasing ionic radius is:
![\[ Ra^(2+) < Sr^(2+) < Ba^(2+) < Mg^(2+) < Be^(2+) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/mga0jtmrpsb3dyqs0xp3eo41zv7djvzemw.png)
b) Cl⁻¹, Mg²⁺, Na⁺¹, P³⁻, Al³⁺, S²⁻:
For ions with different charges, you need to consider both the effect of the charge and the number of energy levels.
1. Consider the charge:
- For a given ion, as the charge increases, the ionic radius generally decreases (more charge pulls the electrons in closer).
- For a given ion with different charges, the higher charge corresponds to a smaller ionic radius.
2. Consider the number of energy levels:
- For ions with the same charge, more energy levels generally result in a larger ionic radius.
Based on these principles, the order of increasing ionic radius is:
![\[ Al^(3+) < Mg^(2+) < Na^(+) < Cl^(-) < S^(2-) < P^(3-) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jt866ox8dx5rr8horjiqsf7qknfwl6ma16.png)
So, the complete order for increasing ionic radius is:
![\[ Al^(3+) < Mg^(2+) < Na^(+) < Cl^(-) < S^(2-) < P^(3-) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jt866ox8dx5rr8horjiqsf7qknfwl6ma16.png)