Answer:
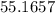 grams/mol of carbon is needed to react with hydrogen gas to produce 59.8 grams of acetylene (C2H2)
grams/mol of carbon is needed to react with hydrogen gas to produce 59.8 grams of acetylene (C2H2)
Step-by-step explanation:
The molecular weight of acetylene is 26.04 g/mol
Molecular weight of carbon is equal to 12.011 g/mol
Two carbon atom produces one acetylene molecule.
Thus, 2 * 12.011 g/mol of carbon is used to produce 26.04 g/mol of acetylene,
Amount of carbon required to produce 59.8 grams of acetylene
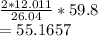 grams/mol
grams/mol
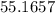 grams/mol of carbon is needed to react with hydrogen gas to produce 59.8 grams of acetylene (C2H2)
grams/mol of carbon is needed to react with hydrogen gas to produce 59.8 grams of acetylene (C2H2)