Answer:
the girl's displacement in both magnitude and direction is 7.81 m at 50.2⁰ North west.
Step-by-step explanation:
Given;
6km due west, and
5km due north
The magnitude of her displacement is calculated by forming a right angled triangle. The hypotenuse side of the triangle is the girl's displacement.
d² = 5² + 6²
d² = 25 + 36
d² = 61
d = √61
d = 7.81 m
The direction of the girl is calculated as;
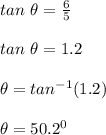
Therefore, the girl's displacement in both magnitude and direction is 7.81 m at 50.2⁰ North west.