Freezing 10g water at -10°C: System gets more ordered (-1.77 kJ/K entropy drop), but surroundings get hotter and less ordered (+1.77 kJ/K entropy gain). Universe's disorder wins.
Calculating Entropy Changes in Water to Ice Conversion
Here's how we can calculate the entropy changes for your scenario:
Given:
* Mass of water (m) = 10 grams = 0.01 kg
* Specific heat of liquid water (cp_water) = 4.2 kJ/kgK
* Specific heat of ice (cp_ice) = cp_water / 2 = 2.1 kJ/kgK
* Latent heat of fusion of ice (Lf) = 335 kJ/kg
* Initial temperature of water (T1) = 20°C
* Final temperature of ice (T2) = -10°C
Calculations:
(i) Total entropy change of the system:
1. Entropy change during cooling of water:
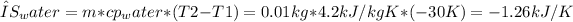
2. Entropy change during phase change (ice formation):
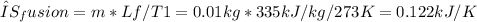
3. Entropy change during heating of ice:
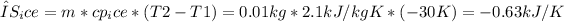
4. Total entropy change of the system:
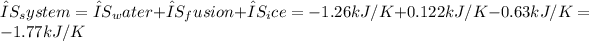
(ii) Net entropy change of the Universe:
Assuming the surroundings are at a constant temperature (i.e., no heat exchange with the environment), the net entropy change of the Universe is equal to the negative of the system's entropy change.
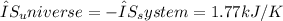
Therefore, the total entropy change of the system is -1.77 kJ/K, and the net entropy change of the Universe is 1.77 kJ/K, indicating an increase in entropy in the surrounding environment due to the heat released during water to ice conversion.
Note:
* These calculations assume ideal conditions and constant specific heats. In reality, specific heats may vary slightly with temperature.