The molar mass of
 is approximately
is approximately
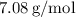 .
.
To determine the molar mass
 of a molecule
of a molecule
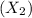 , you can use the formula:
, you can use the formula:
![\[ M = \frac{\text{Mass (g)}}{\text{Amount of substance (moles)}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/17vcbw7r9ial6illz6gdjwff95jcbkgn4u.png)
In this case, you're given the amount of substance in molecules
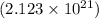 and the mass of
and the mass of
 in grams (0.250 g).
in grams (0.250 g).
Step 1: Determine the number of moles
 using Avogadro's number:
using Avogadro's number:
![\[ \text{Amount of substance (moles)} = \frac{\text{Number of molecules}}{\text{Avogadro's number}} \\\[ n = (2.123 * 10^(21))/(6.022 * 10^(23)) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/6hdln5grrac66syolyh33108tx8hzzl4k2.png)
Step 2: Calculate the molar mass
 :
:
![\[ M = \frac{\text{Mass (g)}}{\text{Amount of substance (moles)}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/17vcbw7r9ial6illz6gdjwff95jcbkgn4u.png)
![\[ M = \frac{0.250 \, \text{g}}{n} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/fgdk81yl1uifwsf4mz783gdo97d8u3jxn4.png)
Plug in the value of n from step 1.
![\[ M = \frac{0.250 \, \text{g}}{(2.123 * 10^(21))/(6.022 * 10^(23))} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/13ab3361eqqyh0bd8ct7lofzmaf84zvt8d.png)
Calculate the result.
The unit for molar mass will be grams per mole (g/mol), which is the standard unit for expressing molar mass.
Performing the calculation:
![\[ n = (2.123 * 10^(21))/(6.022 * 10^(23)) \approx 0.0353 \, \text{mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/cl6m5cdtx7j9rdyl5fu09jgp1fcdba1hge.png)
![\[ M = \frac{0.250 \, \text{g}}{0.0353 \, \text{mol}} \approx 7.08 \, \text{g/mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/q6jhf12xpsejpikf7t84p3inly4abz76tw.png)
So, the molar mass of
 is approximately
is approximately
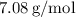 .
.