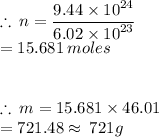Answer:
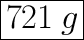
Step-by-step explanation:
The mass of the
 molecules can be found by using the formula:
molecules can be found by using the formula:
m = n × M
where:
m is the mass in grams
M is the molar mass in g/mol
n is the number of moles
From the question:
M = 46.01 g/mol
From the question, the number of moles was not given, however the number of molecules was given. So the number of molecules is then used to find the number of moles by using the formula:
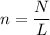
where:
n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question:
N = 9.44 × 10²⁴ molecules