Final answer:
Tripling the temperature of an ideal gas molecule increases its speed by a factor of the square root of three
 , not by tripling the speed, due to the direct proportionality of kinetic energy to the temperature.
, not by tripling the speed, due to the direct proportionality of kinetic energy to the temperature.
Step-by-step explanation:
If the temperature
 of each molecule in an ideal gas is tripled, the speed of the gas molecules does not simply triple. This is because the average kinetic energy of an ideal gas is directly proportional to its absolute temperature.
of each molecule in an ideal gas is tripled, the speed of the gas molecules does not simply triple. This is because the average kinetic energy of an ideal gas is directly proportional to its absolute temperature.
According to the kinetic molecular theory, the kinetic energy
 of an ideal gas is given by,
of an ideal gas is given by,
The equation:
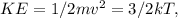
where m is the mass of a molecule, v is the speed of the molecule, k is the Boltzmann constant and T is the absolute temperature in kelvins. When temperature is tripled, assuming the mass of the particles remains constant the kinetic energy of the gas also triples since it is directly proportional to the temperature.
However since
 is proportional to the square of the speed
is proportional to the square of the speed
 , tripling the kinetic energy will only increase the speed of the gas molecules by the square root of three. This means that the speed of a molecule in an ideal gas will increase by a factor of the square root of three
, tripling the kinetic energy will only increase the speed of the gas molecules by the square root of three. This means that the speed of a molecule in an ideal gas will increase by a factor of the square root of three
 , not triple, when the temperature is tripled.
, not triple, when the temperature is tripled.