To balance the equation
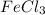 (aq) +
(aq) +
 (aq) →
(aq) →
 (s) + NH4Cl(g), we need to put coefficients in front of the formulas to make sure that the number of atoms on both sides of the equation are equal. The correct answer is option A.
(s) + NH4Cl(g), we need to put coefficients in front of the formulas to make sure that the number of atoms on both sides of the equation are equal. The correct answer is option A.
To balance the first equation,
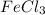 (aq) +
(aq) +
 (aq) →
(aq) →
 (s) +
(s) +
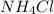 (g), we need to make sure that the number of atoms on both sides of the equation are equal.
(g), we need to make sure that the number of atoms on both sides of the equation are equal.
First, we balance the number of Fe atoms, which is already balanced with 1 Fe on each side. Then, we balance the Cl atoms, which are already balanced with 3 Cl on each side. Next, we balance the N atoms by placing a coefficient of 4 in front of the
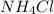 on the right side.
on the right side.
Finally, we balance the H and O atoms by putting a coefficient of 3 in front of the
 on the left side, and a coefficient of 3 in front of the Fe(OH)3 on the right side. The balanced equation is: 3
on the left side, and a coefficient of 3 in front of the Fe(OH)3 on the right side. The balanced equation is: 3
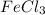 (aq) + 4
(aq) + 4
 (aq) →
(aq) →
 (s) + NH4Cl(g).
(s) + NH4Cl(g).
Therefore, option A is correct.