The heat given off by the sample is approximately 16842.19 J.
To calculate the heat given off, we can use the formula
 , where Q is the heat, m is the mass, c is the specific heat capacity, and
, where Q is the heat, m is the mass, c is the specific heat capacity, and
 is the change in temperature.
is the change in temperature.
Given:
- Mass of water (m) = 3.50 kg
- Change in temperature (
 ) = 1.17 °C
) = 1.17 °C
- Specific heat capacity of water (c) ≈ 4186 J/kg°C
- Heat capacity of the calorimeter (
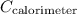 ) = 883 J/°C
) = 883 J/°C
![\[ Q_{\text{water}} = m \cdot c \cdot \Delta T = 3.50 \, \text{kg} \cdot 4186 \, \text{J/kg C} \cdot 1.17 \,{^\circ C} \]](https://img.qammunity.org/2024/formulas/chemistry/college/jyrj866czwdun451gzkbbx865zv90unpxu.png)
![\[ Q_{\text{calorimeter}} = C_{\text{calorimeter}} \cdot \Delta T = 883 \, \text{J/ C} \cdot 1.17 \°C} \]](https://img.qammunity.org/2024/formulas/chemistry/college/21e3j96zqnovt6ty9e2erfo7268hm59k0d.png)
The total heat given off (
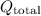 ) is the sum of
) is the sum of
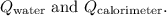
![\[ Q_{\text{total}} = Q_{\text{water}} + Q_{\text{calorimeter}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/msfj037fxqmp7nslmi5z9rmuht67tx1182.png)
![\[ Q_{\text{total}} \approx (3.50 \, \text{kg} \cdot 4186 \, \text{J/kgC} \cdot 1.17 \, \text{C}) + (883 \, \text{J/C} \cdot 1.17 \, \text{C}) \]](https://img.qammunity.org/2024/formulas/chemistry/college/kre5ihpixqkodybgovt42xqjqw36akpoen.png)
![\[ Q_{\text{total}} \approx 16842.19 \, \text{J} \]](https://img.qammunity.org/2024/formulas/chemistry/college/8yhbnxnd0z5u2ci8adwsjyzbh5vx4s6toc.png)