Answer:
The concentration of hydrogen ions in moles per liter (M) is

Explanation:
As we know
The hydrogen ion concentration of any solution at pH is given by
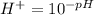
Substituting the given values in above equation, we get -
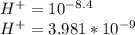 mol/l
mol/l
The concentration of hydrogen ions in moles per liter (M) is
