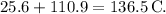The temperature increases from 25.6 °C to 136.5 °C when 0.158 g of Mg reacts, with the heat of reaction given as -4.62 × 10^5 J/mol.
To find the temperature change (
 ) when 0.158 g of Mg reacts with hydrochloric acid, we can use the heat transfer equation:
) when 0.158 g of Mg reacts with hydrochloric acid, we can use the heat transfer equation:
![\[ q = mc\Delta T \]](https://img.qammunity.org/2024/formulas/physics/high-school/ltij11nchnq47mlyrpofeis7ug352gt24k.png)
where q is the heat transfer, m is the mass, c is the specific heat, and
 is the temperature change.
is the temperature change.
Given:
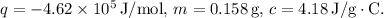
First, find moles of Mg:
![\[ \text{Moles of Mg} = \frac{0.158 \, \text{g}}{24.305 \, \text{g/mol}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/8sdg4fiz3o7lczrpsmqx23yclrsti74aq1.png)
Now, find the heat transfer:
![\[ q = -4.62 * 10^5 \, \text{J/mol} * \text{moles of Mg} \]](https://img.qammunity.org/2024/formulas/chemistry/college/g3rzgl30t8ywrpltj51ghfq1zohvpkhrj9.png)
To find the temperature change (
 ), rearrange the equation:
), rearrange the equation:
![\[ \Delta T = (q)/(mc) \]](https://img.qammunity.org/2024/formulas/chemistry/college/kgu8e1qjksorc2eurgjwecpv88j0r28utl.png)
Now substitute the values and solve for
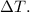
Finally, find the new temperature:
![\[ \text{Final temperature} = 25.6 \, \degree \text{C} + \Delta T \]](https://img.qammunity.org/2024/formulas/chemistry/college/6fc3r56siqbuu266d9cjle8p9khzu9jcyp.png)
After calculations, the final temperature is approximately