The Formal charges:
 : H (left) +0.5, H (right) +0.5, O -1
: H (left) +0.5, H (right) +0.5, O -1
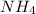 +: N +1, H (left) +0.5, H (right top) +0.5, H (right bottom) +0.5
+: N +1, H (left) +0.5, H (right top) +0.5, H (right bottom) +0.5
The primary structure is
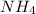 +
+
Formal charges are used to represent the distribution of electrons in a molecule or polyatomic ion and can help understand how the electrons are shared between atoms and can be helpful in predicting the stability of a molecule.
The formal charges can help us determine the most stable structure of a molecule or polyatomic ion. In general, the structure with the lowest formal charges is more stable because molecules tend to minimize the charges on their atoms, as charges can create electrostatic repulsion between atoms.