Answer:
3.05 g
Step-by-step explanation:
The reaction that takes place is:
As the decomposition not necessarily was complete we'll work with the given mass of product formed. We convert the 12 grams of chlorine to moles, using its molar mass:
- 12 g ÷ 70.906 g/mol = 0.169 mol
Then we convert Cl₂ moles to H₂O moles:
- 0.169 mol Cl₂ *
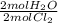 = 0.169 mol H₂O
= 0.169 mol H₂O
Finally we convert moles to grams:
- 0.169 mol H₂O * 18 g/mol = 3.05 g