Final Answer:
This answer is derived by calculating the number of ²³⁹Pu nuclei needed for a 20.0-kT yield, given the energy per fission (200 MeV). The result is 3.38×10²⁵ nuclei, and using the atomic mass of ²³⁹Pu, we find the mass to be 528kg. Therefore, the correct answer is (c) (a) 3.38×10²⁵; (b) 528kg.
Step-by-step explanation:
In nuclear physics, the yield (energy release) from fission reactions can be determined by the equation:
\[ E_{\text{yield}}
![= N * E_{\text{fission}} \]](https://img.qammunity.org/2024/formulas/physics/high-school/3vqakxfxsfgu3w60e846c95p54s69gfczq.png)
where
 is the number of fission events and
is the number of fission events and
 is the energy released per fission. Rearranging for
is the energy released per fission. Rearranging for
 :
:
![\[ N = \frac{E_{\text{yield}}}{E_{\text{fission}}} \]](https://img.qammunity.org/2024/formulas/physics/high-school/nylyz8z9r56vnmetpszpshxxu7m41r0f8f.png)
Substituting the given values, we get:
![\[ N = \frac{(20.0 \, \text{kT}) * (4.184 * 10^(12) \, \text{J/T})}{200 \, \text{MeV}} \]](https://img.qammunity.org/2024/formulas/physics/high-school/tfychk84bhm734ui8e4biimrrzvohxwv18.png)
Solving for
 . This is the number of ²³⁹Pu nuclei that must undergo fission to produce the given yield.
. This is the number of ²³⁹Pu nuclei that must undergo fission to produce the given yield.
The mass of
 nuclei of ²³⁹Pu can be calculated using Avogadro's number
nuclei of ²³⁹Pu can be calculated using Avogadro's number
 and the molar mass of ²³⁹Pu
and the molar mass of ²³⁹Pu
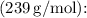
![\[ \text{Mass} = \frac{3.38 * 10^(25) \, \text{nuclei}}{6.022 * 10^(23) \, \text{nuclei/mol}} * 239 \, \text{g/mol} \]](https://img.qammunity.org/2024/formulas/physics/high-school/jjdzg2e7rdo5us9jltdr4m1o1hqma7jcip.png)
The result is approximately 528kg. Therefore, the final answer is (c) (a) 3.38×10²⁵; (b) 528kg.
Therefore, the correct answer is (c) (a) 3.38×10²⁵; (b) 528kg.