Answer:
goodman = 0.694
life of beam = 211597
Step-by-step explanation:
alternating stress = 48 kpsi
mean stress = 24 kpsi
ultimate strength = 100 kpsi
endurance limit = 40 kpsi
goodman:
=
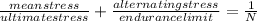
=
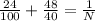
= 0.24 + 1.2 =
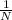
N = 1/1.44
N = 0.694
2. check attachment for diagram
Log(N)-3/3 = log90 - log48/log90 - log40
Log(N)-3/3 = 0.77517
Log N = 5.325509
N = 10^(5.325509)
N = 211597