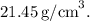Final answer:
The edge length of the face-centered cubic unit cell for platinum is 3.92 Å. The density of platinum is approximately
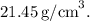
Step-by-step explanation:
*Calculating the Edge Length of a Face-Centered Cubic Unit Cell for Platinum*
In a face-centered cubic (FCC) structure, the relationship between the atomic radius (r) and the edge length (a) of the unit cell is given by the equation

For platinum, with an atomic radius of 1.38 Å, the edge length (a) of the FCC unit cell is calculated as follows:
![\[ a = 2√(2) * 1.38 = 3.92 \, \text{Å} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/vvu2cuca11cnbvqttuaeaoppi0hvnmb3tk.png)
*Calculating the Density of Platinum*
To calculate the density of platinum
 , the formula is used:
, the formula is used:
![\[ \rho = \frac{\text{mass of atoms in a unit cell}}{\text{volume of the unit cell}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/8hch1ulwdpnuny63zgnad6c3egvjjjrxx3.png)
For an FCC structure, there are 4 atoms per unit cell. The mass of a platinum atom (atomic weight = 195.08 u) is converted to grams using Avogadro's number
 .
.
The volume of the unit cell is
 . which, in this case, is
. which, in this case, is
 . Combining these values, we calculate the density of platinum as approximately
. Combining these values, we calculate the density of platinum as approximately