Answer:
T2 = 355.92 Kelvin or 82.92°C
Step-by-step explanation:
Given the following data;
Mass = 567g to kilograms = 567/1000 = 0.567 kg
Quantity of heat = 18,100J
Initial temperature = 15°C to Kelvin = 15 + 273 = 288K
Specific heat capacity of iron = 470j/kg•k
To find the final temperature;
Heat capacity is given by the formula;
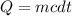
Where;
- Q represents the heat capacity or quantity of heat.
- m represents the mass of an object.
- c represents the specific heat capacity of water.
- dt represents the change in temperature.
Making dt the subject of formula, we have;
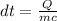
Substituting into the equation, we have;
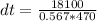
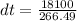
dt = 67.92K
Now, the final temperature T2 is;
But, dt = T2 - T1
T2 = dt + T1
T2 = 67.92 + 288
T2 = 355.92 Kelvin or 82.92°C