For (a) the order is
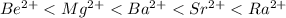 , and for (b) the order is
, and for (b) the order is
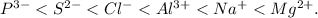
a) **Arranging ions in increasing ionic radius:**
![\[ Be^(2+) < Mg^(2+) < Ba^(2+) < Sr^(2+) < Ra^(2+) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/wo4418iwqkw9m5hxlck12xeiqghaqhn556.png)
Explanation: As we move down a group in the periodic table (from Be to Ra), the ionic radius increases due to the addition of more electron shells.
b) **Arranging ions in increasing ionic radius:**
![\[ P^(3-) < S^(2-) < Cl^(-) < Al^(3+) < Na^(+) < Mg^(2+) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/gd612yvrd8nhf7yk03ke59h7ovv06ytmdq.png)
Explanation: Within a period, as we move from right to left, the ionic radius generally increases because there are fewer electrons, resulting in a smaller effective nuclear charge, leading to a larger radius.