Answer:
the charge carriers have an energy 2.8 10⁻¹⁹ J
Step-by-step explanation:
The energy in a diode is conserved so the energy supplied must be equal to the energy emitted in the form of photons.
The energy of a photon is given by the Planck expression
E = h f
the speed of light, wavelength and frequency are related
c = λ f
we substitute
E =
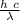
a red photon has a wavelength of lam = 700 nm = 700 10⁻⁹ m
we calculate the energy
E = 6.626 10⁻³⁴ 3 10⁸/700 10⁻⁹
E = 2.8397 10⁻¹⁹J
therefore the charge carriers have an energy 2.8 10⁻¹⁹ J,