Final answer:
The molecular formula for a compound with a formula mass of
 and an empirical formula of
and an empirical formula of
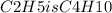 . This is found by calculating the empirical formula mass and dividing the given formula mass by this number, then multiplying the empirical formula by the resulting factor,
. This is found by calculating the empirical formula mass and dividing the given formula mass by this number, then multiplying the empirical formula by the resulting factor,
 .
.
Step-by-step explanation:
The question is aiming to determine the molecular formula for a compound given its empirical formula and formula mass. The empirical formula provided is
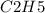 , and the formula mass is
, and the formula mass is
 . First calculate the mass of the empirical formula,
. First calculate the mass of the empirical formula,
which is
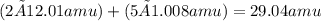 .
.
Next, divide the given formula mass by the empirical formula mass:
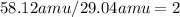 .
.
hus, the molecular formula is found by multiplying the empirical formula by this factor yielding (C2H5)2 or C4H10. Therefore, the molecular formula for the compound is C4H10.