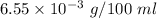Answer:
The right solution is "
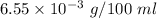 "
"
Step-by-step explanation:
The given values are:
Total pressure,
= 4.79 atm
Mole fraction,
= 78.1
Solubility,
S =
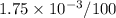
Partial pressure,
P = 1
By using the Henry's law,
⇒
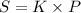
On putting the given values, we get
⇒
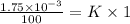
⇒
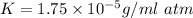 (Henry's constant)
(Henry's constant)
The pressure of nitrogen (
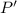 ) will be:
) will be:
=
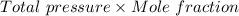
On substituting the above given values, we get
=
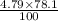
=
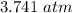
New solubility of nitrogen will be:
⇒
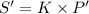
⇒
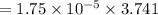
⇒
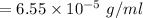
So,
`The solubility of water will be:
=
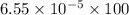
=