Answer:
The mass of air stored in the vessel is 235.34 kilograms.
Step-by-step explanation:
Let supossed that air inside pressure vessel is an ideal gas, The density of the air (
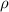 ), measured in kilograms per cubic meter, is defined by following equation:
), measured in kilograms per cubic meter, is defined by following equation:
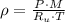 (1)
(1)
Where:
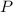 - Pressure, measured in kilopascals.
- Pressure, measured in kilopascals.
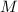 - Molar mass, measured in kilomoles per kilogram.
- Molar mass, measured in kilomoles per kilogram.
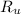 - Ideal gas constant, measured in kilopascal-cubic meters per kilomole-Kelvin.
- Ideal gas constant, measured in kilopascal-cubic meters per kilomole-Kelvin.
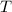 - Temperature, measured in Kelvin.
- Temperature, measured in Kelvin.
If we know that
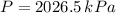 ,
,
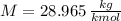 ,
,
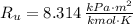 and
and
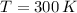 , then the density of air is:
, then the density of air is:
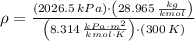
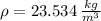
The mass of air stored in the vessel is derived from definition of density. That is:
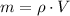 (2)
(2)
Where
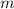 is the mass, measured in kilograms.
is the mass, measured in kilograms.
If we know that
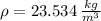 and
and
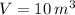 , then the mass of air stored in the vessel is:
, then the mass of air stored in the vessel is:
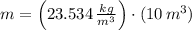
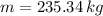
The mass of air stored in the vessel is 235.34 kilograms.