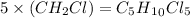Answer:
Step-by-step explanation:
- From the question the empirical formula of the substance is
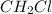 . This means that the actual molecular formula was reduced by a certain factor for the empirical formula to be obtained.
. This means that the actual molecular formula was reduced by a certain factor for the empirical formula to be obtained. - First the empirical formula is multiplied by a variable let's say x and equating it to the actual molar mass of the compound.
- After obtaining the variable, it's then multiplied by the empirical formula to obtain the actual molecular formula.
The molar mass of the compound from the question is 247.3979 g/mol.
![(CH_2Cl)x = 247.3979\\ [12 + (2 * 1) + 35.5)]x = 247.3979 \\ = (12+2+35.5)x = 247.3979 \\]()
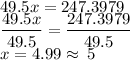
So the actual molecular formula is: