1.
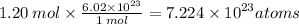
2.
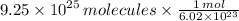
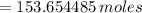
3.
molar mass = 3(24.31) + 127.60 + 5(32.07)
molar mass = 360.88 g
4.
molar mass = 3(58.93) + 2(30.97) + 8(16.00)
molar mass = 366.73 g
5.
molar mass = 98.09
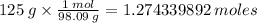
//
I'm too lazy to do the rest but you should get a general idea of how to do it.
Also, round as you wish!