Answer:
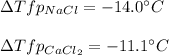
Step-by-step explanation:
Hello!
In this case, since the freezing point depression caused by the addition of a solute, we use the following formula:
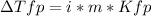
Thus, we first need to compute the molality of each solute, as shown below:

Next, since NaCl has two ionic species, one Na⁺ and one Cl⁻, and CaCl₂ three, one Ca²⁺ and two Cl⁻, the van't Hoff's factors are 2 and 3 respectively, therefore the freezing point depressions turn out:
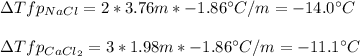
It means that CaCl₂ is still better than NaCl because produces involves a higher melting point for the ice, so it would melt faster.
Best regards!