Answer:
See explanation.
Step-by-step explanation:
Hello!
In this case, when having the cationic and anionic species with the specified charges, in order to abide by the net charge rule, we need to exchange the charges in the form of subscripts and without the sign, just as shown below:
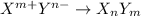
Thus, for all the given combinations, we obtain:
- Y⁻
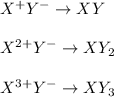
- Y²⁻
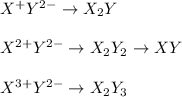
- Y³⁻
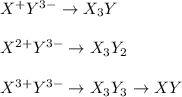
Best regards!