Answer:
a) Same
b) Nitrogen
c) Same
d) Nitrogen
Step-by-step explanation:
a)
The formula for partial pressure of a gas is equal to
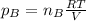
Here nB is the number of moles .
The number of moles for both the gases are same and hence the partial pressure for the two gases will also be same.
b) The greater average velocity is calculated by using following formula
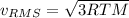
Here M is the molar mass.
Molar mass of nitrogen is greater than the molar mass of xenon and hence nitrogen will have higher greater average velocity
c) As we know, the average kinetic energy of gas particles is dependent on the absolute temperature of gas and if all the gases are at same temperature, their kinetic energy will also be same. Since nitrogen and xenon are at same temperature, their kinetic energy will be same
d) Effusivity is depended directly on the thermal conductivity, density and and the specific heat capacity.
All these three parameters are higher in case of nitrogen. Thus, it will effuse first