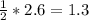Answer:
2.6 moles of HCl will produce moles of H2 =
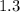
Step-by-step explanation:
Mass of HCl is 36.458 g/mol
Mass of Hydrogen is 1.00794 g/mol
The balanced equation here is
Mg + 2_HCI → _MgCl2 + H2'
2 moles of HCl produces one mole of H2
One mole of HCl will produce moles of H2 =
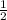
2.6 moles of HCl will produce moles of H2 =